Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Matsumoto, Yoshihiro; Sakai, Seiji; Naramoto, Hiroshi*; Hirao, Norie*; Baba, Yuji; Shimada, Toshihiro*; Sugai, Isamu; Takanashi, Koki; Maeda, Yoshihito
Materials Research Society Symposium Proceedings, Vol.1081 (Internet), 6 Pages, 2008/03
Recently, we have found the appearance of substantial MR ratio (80%) in a C /Co hybrid material. Such the MR ratio cannot be explained enough only by tunnel conduction through Co grains. Therefore, to obtain information about electronic structures of C
/Co hybrid material. Such the MR ratio cannot be explained enough only by tunnel conduction through Co grains. Therefore, to obtain information about electronic structures of C /Co hybrid material is necessary. Absorption spectra of C
/Co hybrid material is necessary. Absorption spectra of C Co are different from that of pristine C
Co are different from that of pristine C . In particular, peak intensity corresponding to
. In particular, peak intensity corresponding to 
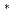 (LUMO)
(LUMO)  C 1s excitation of C
C 1s excitation of C Co is clearly attenuated. In addition, C 1s photoelectron peak of C
Co is clearly attenuated. In addition, C 1s photoelectron peak of C Co slightly shifted to lower binding energy compared to that of pristine C
Co slightly shifted to lower binding energy compared to that of pristine C . These results indicate that 3d electron of Co transfers to
. These results indicate that 3d electron of Co transfers to 
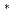 orbital of C
orbital of C and new electronic states are formed in the C
and new electronic states are formed in the C -Co compound. In fact, XPS spectra of valence excitation region also demonstrate the formation of hybrid orbital near the Fermi level due to the coupling of C
-Co compound. In fact, XPS spectra of valence excitation region also demonstrate the formation of hybrid orbital near the Fermi level due to the coupling of C and Co.
and Co.
Yamamoto, Yoshihisa*; Togashi, Hideaki*; Kato, Atsushi*; Suemitsu, Maki*; Narita, Yuzuru*; Teraoka, Yuden; Yoshigoe, Akitaka
Materials Research Society Symposium Proceedings, Vol.1074, p.36 - 40, 2008/00
In this study, we have investigated the bonding structure of ultrathin oxide films on Si(110) surface by real-time SR-PES experiments. Experiments were conducted at surface chemistry end-station settled at BL23SU in SPring-8. The oxidation temperature was 813 K and the O pressure was 1.1
pressure was 1.1 10
10 Pa. As a result, we found that one of the surface core-level shifts in Si 2p spectrum, related to the 1st and the 2nd layer Si atoms, decreases rapidly, coincident with the rapid initial development of the O1s spectrum. This indicates high reactivity of the Si(110)-16
Pa. As a result, we found that one of the surface core-level shifts in Si 2p spectrum, related to the 1st and the 2nd layer Si atoms, decreases rapidly, coincident with the rapid initial development of the O1s spectrum. This indicates high reactivity of the Si(110)-16 2 reconstructed surface with oxygen molecules.
2 reconstructed surface with oxygen molecules.
Kozai, Naofumi; Suzuki, Yoshinori; Nankawa, Takuya; Sakamoto, Fuminori; Onuki, Toshihiko; Francis, A. J.
no journal, ,
To clarify adsorption mechanism of uranium on protein, this study investigated the adsorption of uranium (VI) on silica particles modified with functional groups since functional groups have been considered as the adsorption site of protein. Each of the adsorption edges of uranyl ions occurred in a different pH range: pH  2.5 for the phosphate group-modified particles and 2.5
2.5 for the phosphate group-modified particles and 2.5  pH
pH  3.5 for the amino group-modified particles. Above these pH ranges, the adsorption is almost 100 %. Considering the high content of amino group in protein, amino group can therefore be an important adsorption site for uranyl ions on protein. The decrease of the adsorption on the amino group-modified particles from pH 3.5 to lower pH could be due to the increase of hydronium ions in solution, i.e., the increase of the protonated amino groups.
3.5 for the amino group-modified particles. Above these pH ranges, the adsorption is almost 100 %. Considering the high content of amino group in protein, amino group can therefore be an important adsorption site for uranyl ions on protein. The decrease of the adsorption on the amino group-modified particles from pH 3.5 to lower pH could be due to the increase of hydronium ions in solution, i.e., the increase of the protonated amino groups.